Describe the Electron Density Around Electrophiles
An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. The sulfhydryl group SH of the cysteine is involved in reduction and conjugation reactions that are.

Solved Describe The Electron Density Around An Electrophile Relatively High Electron Density Is Not Relevant For Electrophlles Crelatively Low Unaffected
Electrophiles are Lewis acids Markovnikovs rule can be applied to predict the major product in electrophilic addition reactions of unsymmetrical alkenes with hydrogen halides and interhalogens.

. The tripeptide γ-l-glutamyl-l-cysteinyl-glycine known as glutathione GSH Fig. 1 is the most important low molecular weight antioxidant synthesized in cellsIt is synthesized by the sequential addition of cysteine to glutamate followed by the addition of glycine.
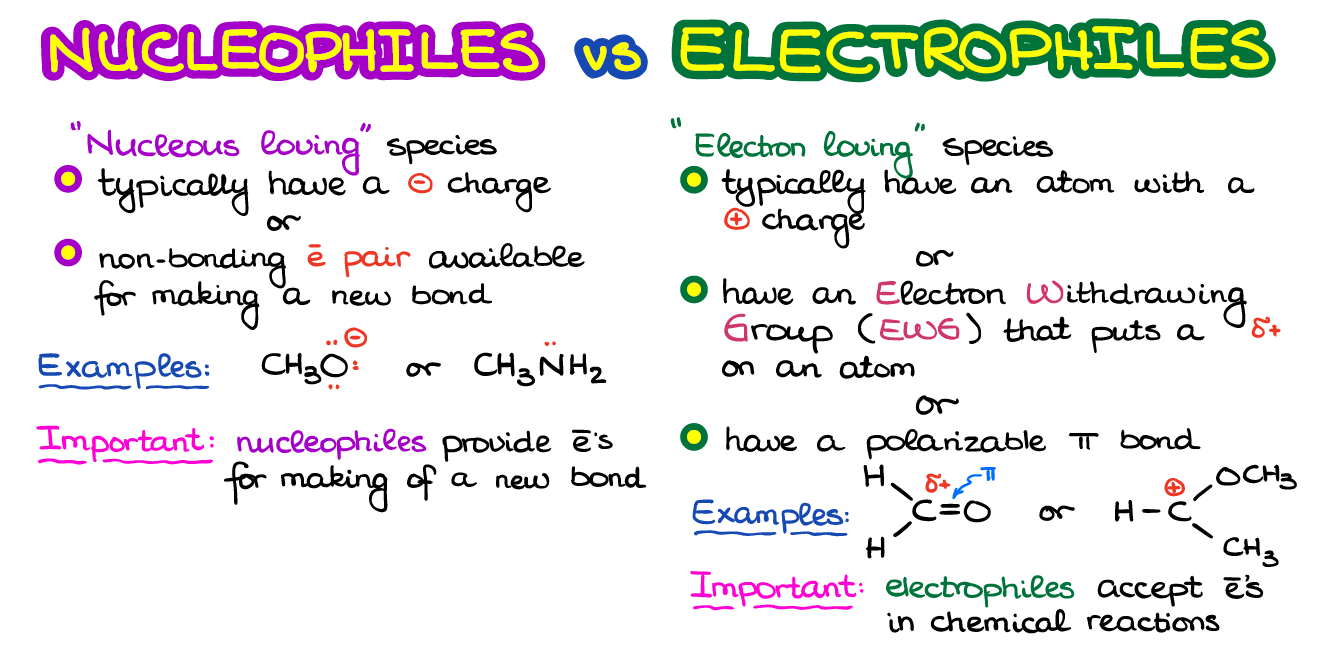
Nucleophiles And Electrophiles Organic Chemistry Tutor

Solved 14 The Electron Density Around Electrophiles Is 1 Not Relevant For Electrophiles 2 Relatively Low 3 Relatively High 4 Unaffected 15 Which Description About Leaving Groups Is Correct 1 Strong Bases Are Good

Solved Describe The Electron Density Around An Electrophile Relatively High Electron Density Is Not Relevant For Electrophlles Crelatively Low Unaffected
No comments for "Describe the Electron Density Around Electrophiles"
Post a Comment